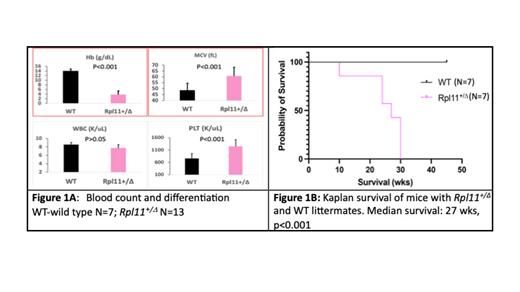
Diamond-Blackfan anemia (DBA) is an inherited bone marrow failure syndrome that presents during early childhood and is characterized by macrocytic anemia, congenital malformations, and predisposition to cancer. More than 90% of DBA patients are diagnosed during their first year of life (median age 12 weeks). The pathogenesis of DBA is linked to loss-of-function mutations in genes encoding ribosomal proteins (RP), although mutations in 3 non-RP genes ( GATA1, TSR2, and HEATR3) have been detected. DBA patients present with a high degree of clinical heterogeneity with varying severity and responses to steroid therapy. DBA provides a unique disease model to study how RP deficiency impacts the phenotype and pathogenesis of ribosomopathies. However, one challenge is the lack of animal models that faithfully recapitulate the clinical features of DBA in terms of disease severity and onset age. Heterozygous loss-of-function mutations in RPL11 are found in 5-20% of DBA patients, but previous mouse models carrying Rpl11 haploinsufficiency have only a mild anemia. Here, we report a novel mouse model of DBA with inducible haploinsufficient expression of Rpl11 which results in a severe macrocytic anemia in juvenile mice. Mx1-Cre Rpl11 +/flox experimental mice were generated by cross-breeding homozygous Rpl11 flox/flox and Mx1-cre breeders. Haploinsufficiency of Rpl11 ( Rpl11 +/Δ) was induced by intraperitoneal injection of polyinosinic-polycytidylic acidto mice with Mx1-Cre Rpl11 +/flox on postnatal day 8 and 10. Diseased mice with Rpl11 +/Δ showed macrocytic anemia at 2 weeks (wks) post-induction with hemoglobin (Hb) < 10 g/dL, eventually dying of severe anemia (Hb< 2 g/mL) by 30 wks (median survival 27 wks, p<0.001, n=7 )( Figure 1). We observed a 50% decreased expression of Rpl11 in blood neucleated cells from Rpl11 +/Δ mice and several characteristic clinical features of DBA. Erythrocyte adenosine deaminase was significantly elevated in the blood of all Rpl11 +/Δ mice when compared to wild type (WT) littermates (median 5 vs 1 EU/g Hg, p<0.001, n=6). Similarly, EPO concentrations were significantly elevated in Rpl11 +/Δ mice compared to WT mice (median 38752 vs 23 pg/mL, p<0.01, n=6). On postmortem examination, the DBA mice displayed splenomegaly. FACS analysis data demonstrated that the differentiation of erythropoiesis is significantly blocked in peripheral blood, bone marrow (BM), and spleen of Rpl11 +/Δ mice compared to WT mice (p<0.05, n = 5). In addition, methylcellulose colony assays revealed that CFU-E colonies were significantly decreased in BM from Rpl11 +/Δ mice in comparison to WT mice (median 23.5 vs 40.5 per 0.2 million BM cells in the presence of EPO at a concentration of 1 unit/mL, p<0.05, n=4). To determine whether BM hematopoietic stem cells/progenitor cells from Rpl11 +/Δ mice can transfer the DBA phenotype, we transplanted bone marrow cells of Rpl11+/ Δ mice (CD45.2+) into sublethally irradiated WT mice (CD45.1+). Our data demonstrate that the BM cells from Rpl11 +/Δ donor mice can induce lethal macrocytic anemia in WT recipient mice. Studies on the molecular mechanisms underlying the dysregulated erythropoiesis in Rpl11 +/Δ mice are ongoing. In conclusion, we have successfully generated the first DBA mouse model that recapitulates the hematologic features of DBA patients. This model provides an ideal tool to study the pathogenesis of DBA and other ribosomopathies.
Disclosures
Glader:Agios Pharmaceuticals, Inc.: Consultancy, Research Funding. Doty:Disc Medicine: Research Funding. Abkowitz:Disc Medicine: Membership on an entity's Board of Directors or advisory committees, Research Funding.